Advertisement|Remove ads.
Bristol Myers Stock Jumps As Wall Street Backs Added Enrollment In Alzheimer’s Trial — Retail Calls It ‘Worth A Play’

- JPMorgan and William Blair pointed to positive elements in Bristol Myers’ updated ADEPT-2 plans.
- The Data Monitoring Committee recommended continuing the study with added enrollment after an interim review.
- Stocktwits traders highlighted renewed attention on Alzheimer’s progress and potential upside into March.
Bristol Myers Squibb (BMY) rose over 4% on Wednesday after analysts pointed to positive elements in the company’s updated plans for its ADEPT-2 Phase 3 study of Cobenfy in Alzheimer’s disease psychosis.
JPMorgan Sees ‘Incremental Positive’
JPMorgan said it was “not completely surprised” after Bristol Myers said it will add more patients to ADEPT-2 following irregularities identified at several clinical trial sites. The firm called the update an “incremental positive,” noting that expectations for ADEPT-2 had been low and that additional enrollment improves the chances of a successful study. JPMorgan said the change does not affect the timing of the FDA filing tied to the remaining ADEPT program readouts and maintained an ‘Overweight’ rating on the shares.
William Blair Cites Potentially Positive Read-Through
William Blair analyst Matthew Phipps noted that ADEPT-2 is now expected to read out by the end of 2026, compared with the company’s prior target of year-end 2025. He said recommendations from the Independent Data Monitoring Committee and the FDA to continue the trial with more patients suggest positive trends. He added that “any non-failure could be good news” following the year-to-date selloff in Bristol Myers shares. William Blair has a ‘Market Perform’ rating.
Bristol Myers Details Reason For Added Enrollment
Bristol Myers said a blinded review of ADEPT-2 identified irregularities related to clinical trial execution at a small number of sites. The company said it excluded patient data from those sites prior to database lock. An independent interim analysis for efficacy and safety was conducted and reviewed by the Data Monitoring Committee, which recommended continuing the study and enrolling additional patients. Bristol Myers said it remains blinded to study data.
The company said readouts from the ADEPT program, including ADEPT-2, ADEPT-1 and ADEPT-4, are expected by the end of 2026. Cobenfy is already approved for schizophrenia in adults and is being evaluated for psychosis associated with Alzheimer’s disease.
Stocktwits Users Point To Alzheimer’s Focus
On Stocktwits, retail sentiment for Bristol Myers was ‘bearish’ amid ‘high’ message volume.
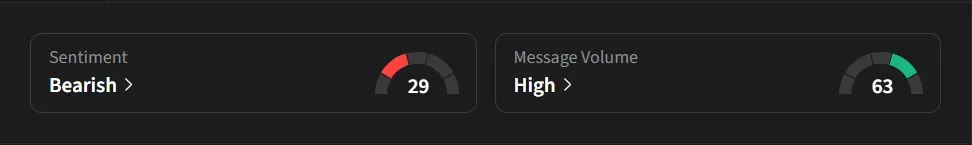
One user said the update underscores how any step toward advancing Alzheimer’s treatment tends to draw attention, adding that Bristol Myers’ decision to restart portions of the work suggests the company wants the data “100% right” after setting aside earlier site results.
Another user said the stock could approach about $60.50 by the end of March, adding that the setup was “worth a play for sure.”
Bristol Myers’ stock has declined nearly 7% so far in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.










/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_140648412_jpg_05552b4117.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_912727778_jpg_3bd44c57ad.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1235943608_jpg_91702ef0ab.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_167578473_jpg_29255f031f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Michael_Burry_jpg_fa0de6ac92.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_winklevoss_1c7de389e0.webp)