Advertisement|Remove ads.
Fate Therapeutics Stock Draws Investor Attention After FDA Grants Special Designation To Its CAR T-Cell Therapy: Retail Stays Optimistic

Shares of Fate Therapeutics, Inc. (FATE) were in the spotlight on Monday morning after the company said that the U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to its FT819.
FT819 is an investigational and human-induced pluripotent stem cell-derived CAR T-cell product candidate in Phase 1 clinical development for treating active moderate to severe lupus, an autoimmune disease that can affect many parts of the body.
CEO Bob Valamehr said that the designation recognises the therapy's potential in treating lupus. The designation allows for early interactions with the FDA and potential priority review of a product’s biologics license application requesting approval to market a biologic product, the company said.
The clinical-stage biopharmaceutical company’s application for the designation included initial clinical safety and activity data from patients treated with FT819 in the ongoing phase 1 clinical trial, it said.
The company added that additional clinical data from the study will be reported later in 2025.
The clinical development of FT819 is supported by a $7.9 million grant from the California Institute of Regenerative Medicine (CIRM).
FATE shares, however, fell by about 13% on Monday morning following the announcement.
On Stocktwits, retail sentiment around Fate Therapeutics rose marginally within the ‘extremely bullish’ territory over the past 24 hours while message volume remained ‘high’.
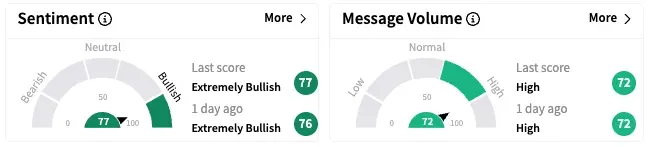
FATE stock is down by about 49% year-to-date and 84% over the past 12 months.
Also See: Soleno Launches Pill For Hyperphagia In Prader-Willi Syndrome Patients But Retail’s Unmoved
For updates and corrections, email newsroom[at]stocktwits[dot]com









/filters:format(webp)https://news.stocktwits-cdn.com/large_btc_x_96cc54b79b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trending_stock_resized_may_jpg_bc23339ae7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_brian_armstrong_coinbase_2_jpg_59a44ebea9.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_jim_cramer_OG_2_jpg_b3d8e3bbe7.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2233918556_jpg_1c5248e175.webp)