Advertisement|Remove ads.
FDA Approves Mirum Pharmaceuticals’ Drug For Rare Liver Disease Patients In Tablet Form: Retail Stays Bullish

Shares of Mirum Pharmaceuticals, Inc. (MIRM) were in the spotlight on Monday after the company announced that the U.S. Food and Drug Administration (FDA) has approved a new tablet formulation of its drug Livmarli for the treatment of cholestatic pruritus in patients with certain liver diseases.
Livmarli is an orally administered ileal bile acid transporter inhibitor. It is used to treat cholestatic pruritus or intense itching caused by impaired bile flow in patients with rare liver diseases called Alagille syndrome (ALGS) and Progressive Familial Intrahepatic Cholestasis (PFIC).
Mirum President Peter Radovich said that the approval for Livmarli in tablet form provides a “meaningful additional treatment option” for patients with ALGS and PFIC.
He said that while younger patients can use liquid dosing, older patients can use a one-tablet-per-dose option. He added that the company has had “tremendous success” with Livmarli since launch.
In 2024, Livmarli's net product sales totaled $213.3 million, accounting for a whopping 63% of the company’s total net product sales.
The tablets are expected to be available in June through Mirum Access Plus pharmacy.
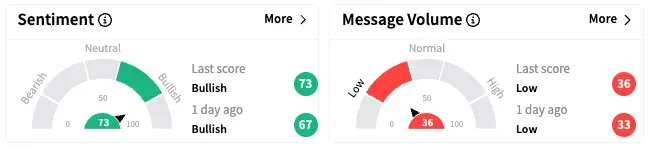
On Stocktwits, retail sentiment around Mirum Pharmaceuticals jumped further into the ‘bullish’ territory while message volume remained at ‘low’ levels.
A Stocktwits user opined that the shares have been forming a “good base” since last year and stand the potential of touching $60.
MIRM stock is down by over 6% year-to-date but has gained over 64% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2250993932_jpg_77e7b26c88.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2209881066_jpg_ebc4b9b217.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_us_japan_jpg_5a4a8c1f81.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227931078_jpg_7ccfff654b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2221559761_jpg_71120b5aaa.webp)