Advertisement|Remove ads.
Gilead Announces Positive Results From Trodelvy-Keytruda Combo Study For Breast Cancer: But Retail’s Unmoved

Gilead Sciences, Inc. (GILD) announced on Monday that its late-stage study showed a combination of its drug Trodelvy with Merck's (MRK) immuno-oncology agent Keytruda significantly improved progression-free survival in patients with a particular aggressive type of breast cancer.
The combination demonstrated better progression-free survival compared to Keytruda and chemotherapy in patients with inoperable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1, the California-headquartered company said.
Triple-negative breast cancer accounts for approximately 15% of all breast cancers and has a higher chance of recurrence.
Gilead Chief Medical Officer Dietmar Berger stated that the findings are the first to demonstrate the transformative potential of an antibody-drug conjugate (Trodelvy) in combination with an immuno-oncology agent in early treatment lines of metastatic breast cancer.
“For patients with this difficult-to-treat type of breast cancer, these results potentially offer a new pathway that may redefine their treatment options,” Berger said.
The use of Trodelvy plus Keytruda in patients with previously untreated PD-L1+ metastatic TNBC is investigational, and the safety and efficacy of this use have not been established, the company highlighted.
It added that the safety profile of Trodelvy plus Keytruda in the phase-3 study was consistent with the known safety profile of each agent.
The late-stage study enrolled 443 patients, and its detailed results will be discussed with regulatory authorities, the company said. Gilead will also continue to monitor overall survival in patients treated with the combination.
Gilead entered a collaboration with Merck & Co. to investigate the combination of the two drugs in 2021.
On Stocktwits, retail sentiment around Gilead is unmoved within the ‘bearish’ territory over the past 24 hours, while message volume remained ‘low’.
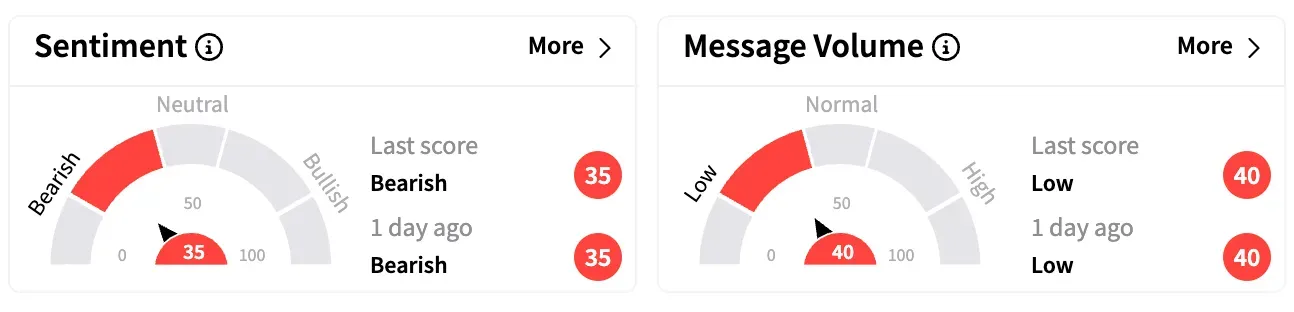
GILD stock is up approximately 13% year-to-date and 55% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com










/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Visa_resized_82d951e81e.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1218279776_jpg_d381694a09.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2199360480_jpg_41abd97106.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Palantir_jpg_d29fd424cd.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2217223717_jpg_e05dddbc9f.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_sea_ltd_jpg_b4cc09a88d.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)