Advertisement|Remove ads.
ImmunityBio Surges On FDA Green Light For Expanded Bladder Cancer Vaccine Access — Retail Buzz Intensifies
Shares of ImmunityBio Inc. surged 16.4% on Wednesday, marking their best session in over a month, and extended gains in after-hours trading.
The rally followed the company’s announcement that the U.S. Food and Drug Administration (FDA) has authorized an expanded access program. This program will provide patients in the U.S. with a critical alternative source of BCG, a standard-of-care treatment for bladder cancer.
ImmunityBio said that Merck’s TICE BCG shortages have created a significant hurdle for bladder cancer treatment.
A recent Sermo survey of 100 U.S. urologists found that 57% could not treat patients over the past year due to a lack of access to TICE BCG.
The Serum Institute of India, the world’s largest vaccine manufacturer by volume, developed the alternative BCG source.
In European clinical trials for bladder cancer, ImmunityBio said its recombinant BCG vaccine demonstrated strong immunogenicity with CD8+ and CD4+ T cell stimulation and improved safety compared to earlier BCG strains.
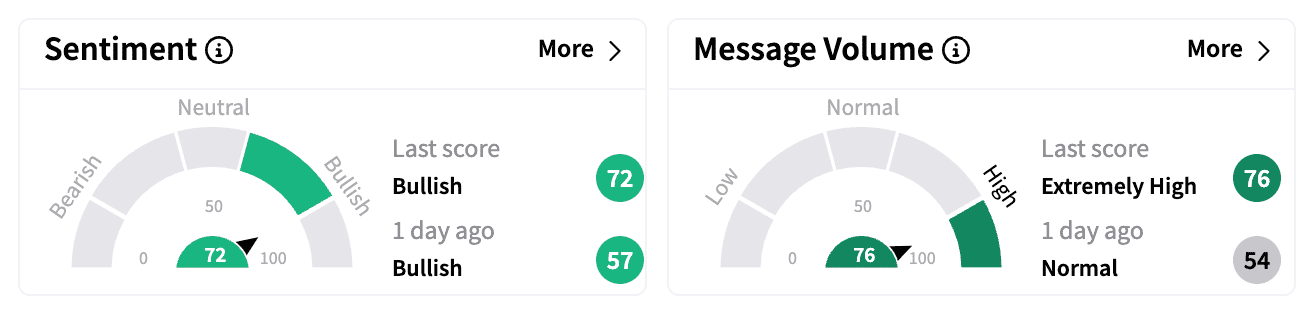
On Stocktwits, sentiment for ImmunityBio turned even more bullish, with message volume surging by 450% by the end of Wednesday.
Retail traders expressed enthusiasm, with one user calling the news “as good as it can get other than a bid” and another speculating that ImmunityBio’s combination therapy with Anktiva could become the new standard of care, replacing Merck’s BCG.
Shares of ImmunityBio have gained more than 50% so far this year, though short interest, according to Koyfin, climbed from 6.4% at the start of 2025 to 7.7% by the end of last week.
For updates and corrections, email newsroom[at]stocktwits[dot]com.














/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227669377_jpg_9a115c3623.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2227231004_jpg_0de480c6f4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2254924041_jpg_892ccf911d.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_1234736300_jpg_881ee00045.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2218899763_jpg_5d8b51f97d.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Trump_golden_dome_resized_jpg_38d699de1f.webp)