Advertisement|Remove ads.
Outlook Therapeutics Tanks Over 60% On Another FDA Blow – Here’s How Retail Is Reacting

- The FDA said it cannot approve the application in its present form for the treatment of wet age-related macular degeneration.
- This marked the second rejection for Outlook’s drug for wet AMD treatment in less than six months.
- The CEO said, “We are disappointed and disagree with this decision.
Shares of Outlook Therapeutics (OTLK) slumped more than 60% on Friday after it failed to secure U.S. Food and Drug Administration approval for its drug Lytenava, which is intended to treat wet age-related macular degeneration (wet AMD), a chronic eye disorder.
This marked the second rejection for Outlook’s drug for wet AMD treatment in less than six months. In August, the FDA declined to approve the drug, citing insufficient evidence of effectiveness, and recommended that the company submit additional data to support its application.
In the Complete Response Letter (CRL), the FDA noted that the additional mechanistic and natural history data provided in the biologics license application (BLA) resubmission do not alter the previous review conclusion, adding that the FDA cannot approve the application in its present form for the treatment of wet age-related macular degeneration.
“We are disappointed and disagree with this decision, but we remain fully committed to taking all necessary steps to receive approval in the United States,” Bob Jahr, Chief Executive Officer of Outlook Therapeutics, said in a statement.
Lytenava has been granted marketing authorization by the European Commission in the EU and marketing authorization by the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK for the treatment of wet AMD.
Age-related macular degeneration causes damage to the macula, a small area near the center of the retina, which is the part of the eye responsible for sharp, central vision. It is common among people aged 50 and older.
How Did Stocktwits Users React?
Retail sentiment around OTLK trended in ‘extremely bullish’ territory amid ‘extremely high’ message volumes.
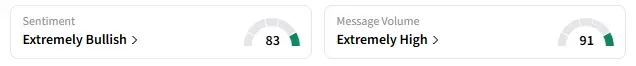
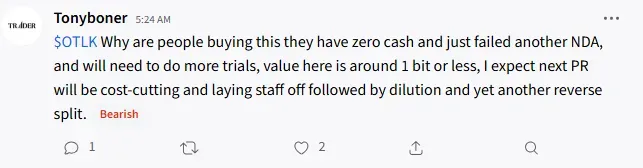
However, the latest blow from the FDA has disappointed retail traders, with one user saying, “Even if they have money, they have proven approval is beyond their abilities.”

The FDA disapproval prompted users to believe the company would do cost-cutting. With one user saying to expect the next PR to be cost-cutting and layoffs, followed by dilution, and yet another reverse split.
Shares of Outlook Therapeutics were down nearly 17% in 2025.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_market_OG_2_jpg_d58f0a637e.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_supermicro_resized_jpg_95d12828d5.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_ray_dalio_resized_jpg_d2f1d535bc.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_pharma_stock_jpg_490939e580.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_jamie_dimon_jpmorgan_jpg_cbdd07fa63.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Git_Lab_resized_49b70b74d0.jpg)