Advertisement|Remove ads.
Pfizer’s Hemophilia Drug Meets Goals In Late-Stage Trial In Patients With Certain Antibodies

Pfizer Inc. (PFE) on Thursday said that its therapy Hympavzi demonstrated improvement in bleeding outcomes for adults and adolescents living with hemophilia A or B with inhibitors in a late-stage study.
Hemophilia A and B are inherited bleeding disorders caused by deficiencies in clotting factors. Inhibitors are antibodies that develop in some individuals with hemophilia, which make it harder to control bleeding. These antibodies neutralize factor replacement therapies and render them ineffective in hemophilia patients.
The late-stage study by Pfizer demonstrated that preventative treatment with Hympavzi resulted in a statistically significant and clinically relevant reduction in annualized bleeding rate (ABR) of treated bleeds in people living with severe hemophilia A or hemophilia B with inhibitors.
During the study, 48 people living with hemophilia were treated with Hympavzi and observed to have a 93% reduction in ABR in 12 months. The drug also demonstrated a reduction in spontaneous bleeds, joint bleeds, target joint bleeds, and total bleeds, the company said.
No deaths or thromboembolic events were reported in the study, the company added.
Pfizer said that it intends to discuss the data with regulators with the goal of initiating a regulatory filing for the drug.
Hympavzi has already received regulatory approvals in the U.S. for patients living with hemophilia A or hemophilia B without inhibitors.
However, according to Pfizer, of the more than 800,000 people in the world living with hemophilia A or hemophilia B, approximately 20% of people with hemophilia A and 3% of people with hemophilia B are unable to continue taking factor replacement therapies because they develop inhibitors.
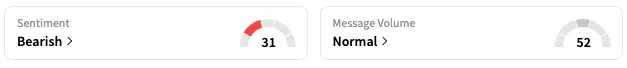
On Stocktwits, retail sentiment around Pfizer stayed unmoved within ‘bearish’ territory over the past 24 hours while message volume remained at ‘normal’ levels.
PFE stock is down by over 8% this year and by over 11% in the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_122032465_jpg_9592f3bcfd.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Novavax_building_93bfe3bf8c.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_stock_price_bitcoin_OG_jpg_d43a7edd5e.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2227994915_jpg_357c625963.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1470886126_jpg_f11dd80298.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262161352_jpg_832967dbca.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)