Advertisement|Remove ads.
Phio Pharmaceuticals Stock Soars On Clearance To Escalate Dose In Skin Cancer Treatment Trial: Retail’s Thrilled

Shares of Phio Pharmaceuticals Corp. (PHIO) traded 63% higher on Wednesday after the company announced that a Safety Monitoring Committee (SMC) recommended escalating the dose in the clinical trial for its lead product candidate, PH-762.
The dose-escalating clinical trial aims to evaluate the safety and tolerability of neoadjuvant use of intratumoral PH-762 in certain skin cancers. The trial assesses tumor response and determines the recommended dose for further study of the product candidate.
PH-762 is an Intasyl compound that silences the PD-1 gene implicated in various forms of skin cancer.
CEO Robert Bitterman said the company is optimistic the clinical trial will continue to demonstrate that PH-762 may present a viable non-surgical alternative to existing modes of therapy for skin cancer.
The company said that two of four patients diagnosed with cutaneous squamous cell carcinoma and enrolled in the second cohort of the trial had 100% tumor clearance upon excision.
While one had a 90% tumor clearance, the fourth patient maintained stable disease, the company said.
Three patients were enrolled in the third cohort of the trials. The injections were well tolerated with no serious adverse events, the company said.
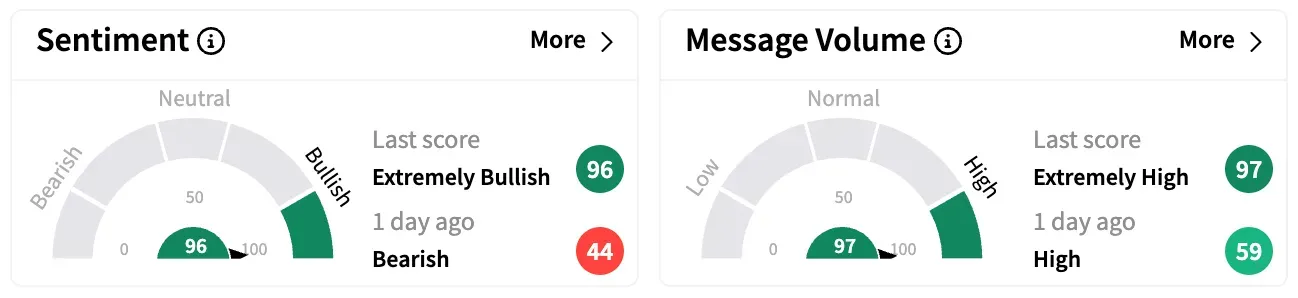
On Stocktwits, retail sentiment around Phio Pharmaceuticals rose from ‘bearish’ to ‘extremely bullish’ while message volume jumped from ‘high’ to ‘extremely high’ over the past 24 hours.
PHIO shares rose as high as $2.73 on Wednesday before paring gains to around $1.50.
The stock fell by over 13% this year and by over 73% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.












/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1445160636_jpg_9759816169.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_opendoor_OG_jpg_55300f4def.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_federal_reserve_jpg_7298dc8578.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_stock_generic_resized_jpg_3444b70edf.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_snap_resized_jpg_9672f61595.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_stock_rising_resized_f17852d7aa.jpg)