Advertisement|Remove ads.
SELLAS Life Sciences Draws Retail Buzz On SLS009 Trial Momentum – Stock Soars In Pre-Market

- SELLAS’s Phase 2 clinical trial for its investigational drug SLS009 for acute myeloid leukemia showed positive results.
- Across the treated group, the overall response rate (ORR) reached 46%.
- For the cohort with exactly one earlier line of treatment, the ORR climbed to 58%.
SELLAS Life Sciences Group, Inc. (SLS) stock came into the spotlight on Monday morning after encouraging results from its Phase 2 clinical trial for its investigational drug SLS009.
The trial involved combining SLS009 with azacitidine (AZA) and venetoclax (VEN) in patients with relapsed or refractory acute myeloid leukemia with myelodysplastic-related changes (AML-MR).
What Did Stocktwits Users Say?
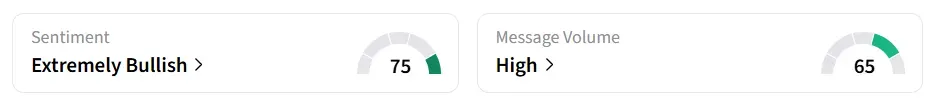
SELLAS’ stock traded over 8% higher in Monday’s premarket. On Stocktwits, retail sentiment around the stock improved to ‘extremely bullish’ from ‘bullish’ the previous day amid ‘high’ message volume.
A Bullish Stocktwits user said there are many situations where Galinpepimut-S (GPS)
and SLS009 can be used alongside venetoclax and azacitidine, creating a powerful multi-drug approach to wipe out AML.
GPS, SELLAS’ lead product candidate, is an immunotherapeutic that targets the Wilms Tumor 1 (WT1) protein.
Another user said they always prefer late-stage biotechs to have at least one more solid product or indication in development.
Encouraging Response Rates
In the study, 35 evaluable individuals, most with high-risk disease marked by adverse-risk genetics, received SLS009 intravenously at 30 mg twice weekly, alongside AZA/VEN.
Across the treated group, the overall response rate (ORR), including complete remission (CR), complete remission with incomplete hematologic recovery (CRi), and morphologic leukemia-free state (MLFS), reached 46%.
Patients who had received minimal prior therapy experienced a median overall survival (mOS) of 8.9 months, far exceeding historical survival expectations of roughly 2.5 months in this patient subgroup. For the cohort with exactly one earlier line of treatment, the ORR climbed to 58%.
SLS stock has gained over 67% year-to-date.
Also See: Why IBM May Want Confluent So Badly: 3 Charts Reveal AI Play's Promise
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1232171389_jpg_55d81c88fb.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Apple_jpg_b7abd92483.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_crypto_atm_OG_jpg_ab7e4567eb.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2244602965_jpg_cba2d012d5.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_novaxovid_novavax_resized_jpg_3a4b0527ae.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_ripple_OG_jpg_e47a5108f1.webp)