Advertisement|Remove ads.
Why Did AGIO Stock Surge 14% Today?

- BofA raised AGIO’s price target to $34 from $32, while maintaining a ‘Buy’ rating, according to The Fly.
- The approval is based on results from the global Phase 3 ENERGIZE and ENERGIZE-T trials, which involved 452 patients.
- Agios expects AQVESME to be available in the U.S. by late January 2026.
Shares of Agios Pharmaceuticals (AGIO) jumped 14% on Wednesday after the U.S. Food and Drug Administration (FDA) approved its oral therapy for anemia in adults with alpha- or beta-thalassemia, an inherited blood disorder.
The FDA approval also prompted BofA to raise AGIO’s price target to $34 from $32, while maintaining a ‘Buy’ rating, according to The Fly. According to the company, AQVESME oral therapy is currently the only FDA-approved medicine for anemia in both non-transfusion-dependent and transfusion-dependent forms of thalassemia.
AQVESME Test Details
The approval is based on results from the global Phase 3 ENERGIZE and ENERGIZE-T trials, which involved 452 patients. Across both studies, AQVESME met all primary and key secondary endpoints, with improvements in hemolytic anemia and quality of life compared with placebo. Patients treated with AQVESME showed significant increases in hemoglobin levels, reduced transfusion burden, and improved fatigue.
During the trials, five patients receiving AQVESME experienced adverse reactions suggestive of hepatocellular injury (HCI), with two of them requiring hospitalization. These events occurred within the first six months of treatment and resolved after stopping the drug.
To manage this risk, the FDA approved AQVESME with a Risk Evaluation and Mitigation Strategy (REMS) program, which includes regular liver testing. Agios expects AQVESME to be available in the U.S. by late January 2026.
How Did Stocktwits Users React?
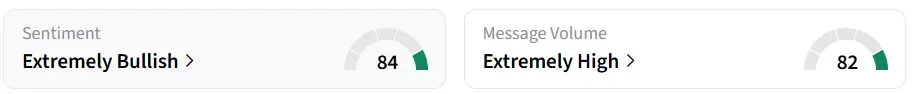
Retail sentiment shifted to ‘extremely bullish’ from ‘bearish’ a day earlier, amid ‘extremely high’ message volumes. AGIO was among the top trending tickers on the platform.
One user sees accumulation but wants higher trading volumes to confirm demand.
Year-to-date, AGIO stock has declined 25%.
Read also: Omeros Stock Garners High Retail Attention Ahead Of FDA Verdict For Life-Saving Drug
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2242061511_jpg_742d610600.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_eli_lilly_hq_resized_af4cc05fd5.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2204154647_jpg_b295df5f6b.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_MSTR_caaa0be909.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Anushka_Basu_make_me_smile_in_the_picture_b92832aa_af59_4141_aacc_4180d2241ba8_1_2_png_1086e0ed8c.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_atomera_logo_resized_97a56614c5.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2250240969_jpg_dd9be8c5ea.webp)