Advertisement|Remove ads.
Eli Lilly Stock Rises After Late-Stage Data Shows Longer Survival With Breast Cancer Drug

- The study showed benefits beyond survival, including slower disease progression in certain patient groups.
- Combining the drug with Abemaciclib extended key treatment timelines compared with using it alone.
- Lilly reported no new safety issues and has submitted the combination data to U.S. regulators.
Shares of Eli Lilly rose over 1% on Friday after the company unveiled updated results from a late-stage study, which showed its breast cancer drug Imlunestrant helped patients live longer and delayed disease progression in people with an advanced form of breast cancer.
In patients whose cancer carried an ESR1 mutation, Imlunestrant, used on its own, reduced the risk of the disease worsening or death by 38% compared with standard hormone therapy.
Patients lived a median of 34.5 months on Imlunestrant, around 11 months longer than those receiving standard treatment, Lilly said, although the result did not meet the strict statistical threshold required for formal significance.
Combination Treatment Shows Strong Benefit
Imlunestrant also showed improved outcomes when combined with Abemaciclib, with median progression-free survival nearly doubling to 10.9 months compared with 5.5 months for Imlunestrant alone. The combination also extended median time to chemotherapy by more than a year and showed a favourable overall survival trend, Lilly said.
In patients with ESR1-mutated disease, the combination helped keep the cancer under control for about 11 months on average. Most patients in this group had already been treated with similar cancer medicines.
Komal Jhaveri of Memorial Sloan Kettering Cancer Center, one of the study’s principal investigators, said “the median progression-free survival of 11 months is among the longest we’ve seen in this population, and just as importantly, patients are living longer without needing chemotherapy.”
Lilly said no new safety issues were identified with longer follow-up. Studies are continuing to track long-term survival, and the drug is also being tested in people with earlier-stage breast cancer.
The results follow the recent U.S. approval of Imlunestrant as a single treatment. Lilly said it has now submitted data on the combination treatment to U.S. regulators for review.
How Did Stocktwits Users React?
On Stocktwits, retail sentiment for Eli Lilly was ‘bullish’ amid ‘normal’ message volume.
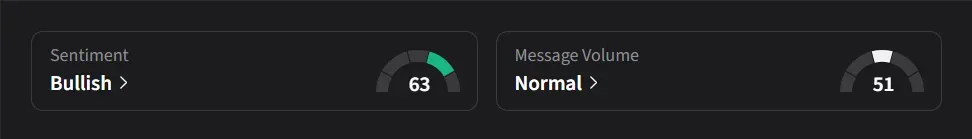
Eli Lilly’s stock has risen 33% so far this year.
Get updates to this developing story directly on Stocktwits.
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2218096416_jpg_9d469a2ec6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_sealsq_stock_market_representative_resized_b05435011f.jpg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Getty_Images_2229019640_jpg_c6006d7238.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_adam_smigielski_K5m_Pt_O_Nmp_HM_unsplash_f365ee93f4.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_trump_jpg_fc59d30bbe.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1190665957_jpg_92088206ed.webp)