Advertisement|Remove ads.
UroGen Stock Slumps After FDA Advisory Committee Flag Concerns On Its Investigational Drug: But Retail’s Unruffled

Shares of biotech company UroGen Pharma Ltd. (URGN) plummeted 48% on Wednesday noon after the company said that a committee of the U.S. Food and Drug Administration (FDA) voted in opposition to a favorable benefit-risk profile for its investigational drug UGN-102, an intravesical solution for the treatment of a recurrent form of bladder cancer.
CEO Liz Barrett said the company is "disappointed" by the outcome of the meeting of FDA’s Oncologic Drugs Advisory Committee (ODAC), but added that the company believes in the clinical data in support of the drug.
Low-grade intermediate-risk nonmuscle-invasive bladder cancer has no FDA-approved therapies, the CEO said, while adding that it is looking forward to working with the FDA as they complete their review of its new drug application (NDA).
The FDA considers independent advice from ODAC, which weighs down the possibility of approval. The FDA is expected to conclude its review of the application by June 13.
UGN-102 for intravesical solution is an innovative drug formulation of mitomycin, an antibiotic used as a drug in cancer treatment. It stops or slows the growth of cancer cells and other rapidly growing cells by damaging their DNA.
The company said the adverse impacts identified with the treatment in its late-stage trial, such as urine retention, are typically manageable in routine urologic practice.
UroGen submitted its NDA to the FDA in August 2024. If approved in June, the company initially said it would make the product available in July.
On Stocktwits, retail sentiment around UroGen remained unchanged within the ‘extremely bullish’ territory over the past 24 hours while message volume stayed put at ‘extremely high’ levels.
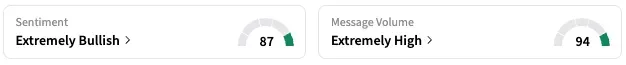
URGN stock is down by about 64% this year and nearly 71% over the past 12 months.
Also See: Shopify Bets On Gen-AI With New AI Tools To Simplify Store Creation But Stock Drops
For updates and corrections, email newsroom[at]stocktwits[dot]com.









/filters:format(webp)https://news.stocktwits-cdn.com/large_qatarenergy_jpg_907aa26daf.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Rounak_Author_Image_7607005b05.png)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2262161352_jpg_832967dbca.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/shivani_photo_jpg_dd6e01afa4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2243817419_jpg_fd782b2997.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2191224409_jpg_fd3e69e2d7.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/vivekkrishnanphotography_58_jpg_0e45f66a62.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2229305833_jpg_f9b80a181a.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Tom_Lee_9782f9c21f.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)