Advertisement|Remove ads.
GLP-1 Showdown In India: Eli Lilly’s Mounjaro Pen Approved Just Days After Novo’s Wegovy Launch
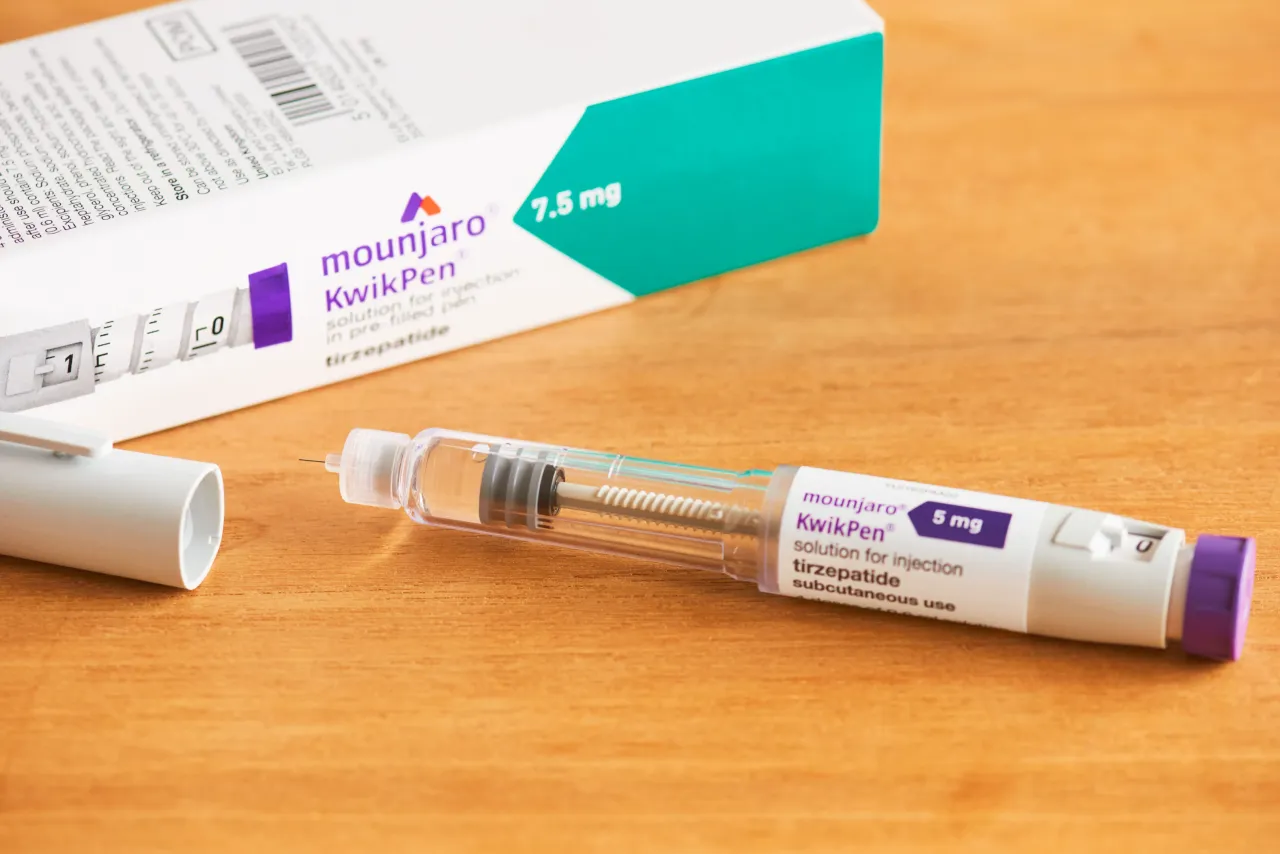
India’s health regulator has approved Eli Lilly’s pre-filled pen version of Mounjaro, expanding its availability across six dosage strengths.
The once-weekly injector, known as Mounjaro KwikPen, now covers doses ranging from 2.5 mg to 15 mg, according to a Reuters report.
Lilly had launched the drug in India earlier this year in vial form, limited to just two strengths.
Just this week, Novo Nordisk rolled out its own weight-loss drug, Wegovy, in India, also in a pen-based format.
Both drugs are in the GLP-1 agonist class, which mimics hormones in the gut to regulate appetite and blood sugar levels.
The drug class has been a global sensation, with demand so high that manufacturers have had trouble keeping up.
India is a strategic battleground for both companies.
The country is dealing with a rapid rise in obesity and diabetes cases, with research suggesting that more than one in ten adults could be obese by 2035.
That makes it one of the most promising and competitive markets for this new generation of weight-loss drugs.
Local pharma giants aren’t standing still.
Indian drugmakers Sun Pharma, Biocon, Zydus, Cipla and Dr. Reddy’s are among those developing cheaper versions of semaglutide, the active ingredient in Wegovy.
The patent will likely expire next year in India, which would open the way for generic versions.
Sun Pharma is taking a different route by developing its own GLP-1 candidate, utreglutide, which it expects to bring to market within five years.
Meanwhile, Cipla has expressed interest in partnering with Lilly to co-market Mounjaro in India.
For updates and corrections, email newsroom[at]stocktwits[dot]com.













/filters:format(webp)https://news.stocktwits-cdn.com/large_Data_center_jpg_5f0fa8e828.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_4530_jpeg_a09abb56e6.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_bitcoin_OG_oct23_jpg_588046d0a9.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/Prabhjote_DP_67623a9828.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_nio_battery_swap_jpg_de98f34bea.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/IMG_8805_JPG_6768aaedc3.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_michael_saylor_strategy_2013_resized_jpg_e358c15fd4.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1760545615_jpg_9507fd561a.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/IMG_9209_1_d9c1acde92.jpeg)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_trader_stock_chart_resized_861d098b1f.jpg)