Advertisement|Remove ads.
Verastem’s Combination Therapy For Ovarian Cancer Gets FDA Approval: Retail’s Pleased

The U.S. Food and Drug Administration on Thursday granted accelerated approval to Verastem Oncology’s (VSTM) combination therapy for adult patients with a particular type of ovarian cancer.
The combination of Avutometinib capsules and Defactinib tablets, under the brand name Avmapki Fakzynja Co-pack, can now be used to treat patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have received prior treatment.
LGSOC is a rare ovarian cancer which is highly recurrent and less sensitive to chemotherapy compared to high-grade serous ovarian cancer. Approximately 6,000-8,000 women in the U.S. and 80,000 worldwide are living with this disease, as per Verastem.
Avmapki Fakzynja Co-pack is the first and only FDA-approved medicine for this disease, Verastem said, while adding that the oral medicine pack is expected to be available for adult patients in the U.S. in one week.
The FDA previously granted priority review to Verastem’s drug application and also designated it a breakthrough therapy and orphan drug.
Late last month, the company announced a $75 million private placement. The proceeds would be used to fund the launch of the combination therapy, among other things.
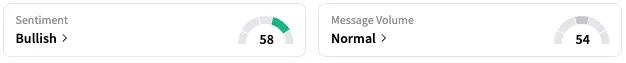
On Stocktwits, retail sentiment around Verastem rose from ‘neutral’ to ‘bullish’ over the past 24 hours while message volume remained at ‘normal’ levels.
The stock is trading 3% higher on Thursday afternoon after a trading halt.
VSTM stock has gained by about 6% this year but has fallen by about 39% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.











/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_fiverr_resized_b6733a31a5.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/Aashika_Suresh_Profile_Picture_jpg_2acd6f446c.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_2240244443_jpg_6b67e8f303.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/unnamed_jpg_9dff551b50.webp)
/filters:format(webp)https://st-everywhere-cms-prod.s3.us-east-1.amazonaws.com/large_Crowdstrike_logo_resized_cce5c5379f.jpg)
/filters:format(webp)https://news.stocktwits-cdn.com/large_Getty_Images_1445160636_jpg_9759816169.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_opendoor_OG_jpg_55300f4def.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/large_federal_reserve_jpg_7298dc8578.webp)
/filters:format(webp)https://news.stocktwits-cdn.com/jaiveer_jpg_280ad67f36.webp)